Global’s Medical Writing Capabilities
Our Regulatory CMC Strategists develop sound and defendable strategies to help clients navigate the complex regulatory landscape.
Our CMC Technical Team provides process and analytical support and program development guidance.
Our Quality Professionals can help maintain compliance with phase-appropriate cGMPs from early development through commercialization.
Our experienced Authoring Teams produce high-quality submission and technical documents.
About the Client
Client C developed autologous cell therapy derived from peripheral blood mononuclear cells (PBMCs), activated and expanded ex vivo with an indication for advanced-stage cancer under compassionate use and early-phase clinical trial setting. The manufacturing time was approximately 48 hours from leukapheresis to final formulation with an administration mode of fresh (non-cryopreserved) infusion within 6 hours post-manufacture.
About the Project
Challenge: Fresh administration does not permit completion of traditional 14-day sterility testing prior to patient dosing. The client looked to compare the testing strategy and regulatory considerations for fresh, same-day administered autologous product, with no sterility results at the time of administration as well as cryopreserved product, where full sterility and other release criteria are available before administration.
Fresh Product – Constraints and Additional Testing
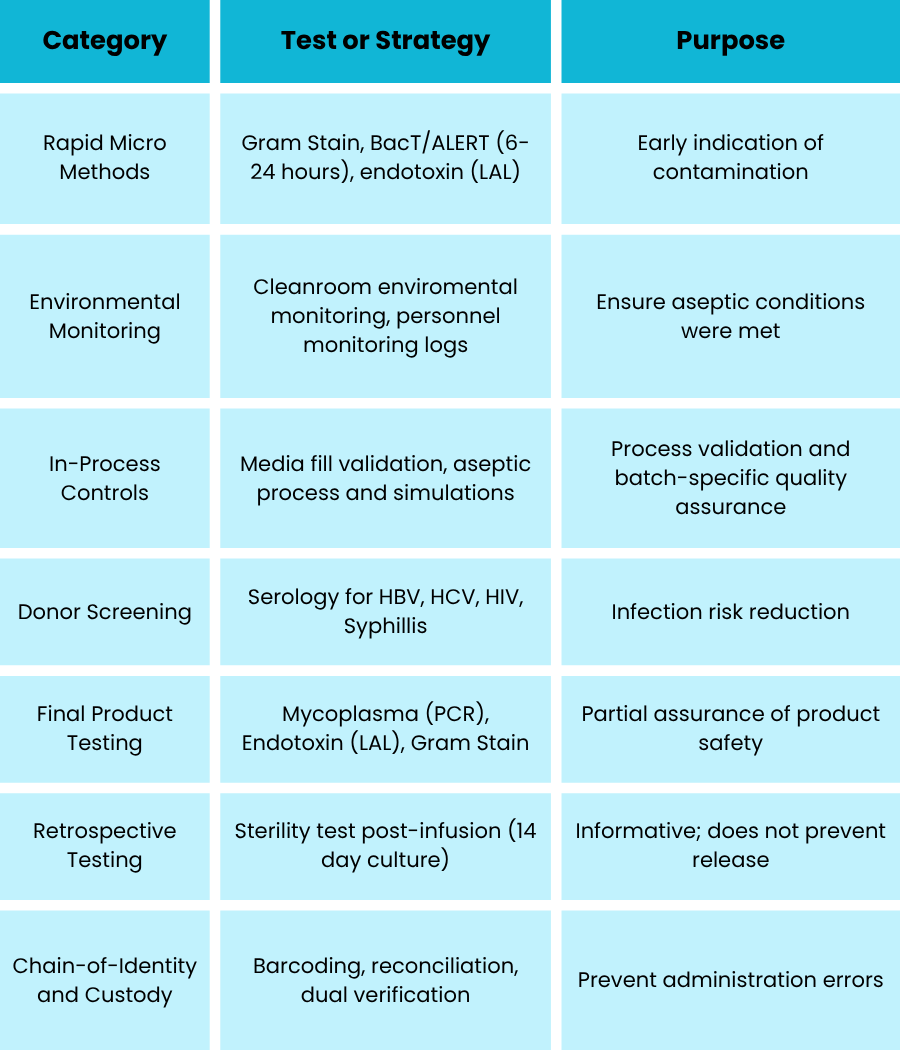
Challenge: Sterility testing not available before administration.
- Traditional sterility testing (USP <71>) takes 14 days
- Fresh product has a 6-to-8-hour shelf-life post-manufacture
- Cannot be held back for test results
Additional tests and risk mitigation required - see table on following page for details.
Regulatory Strategy
- Risk-based approach (per FDA and EMA guidance on advanced therapies)
- Justification for release without sterility based on:
- Aseptic process validation
- Rapid testing results
- Clinical need and autologous nature
- Label includes: "Sterility testing in progress at time of release"
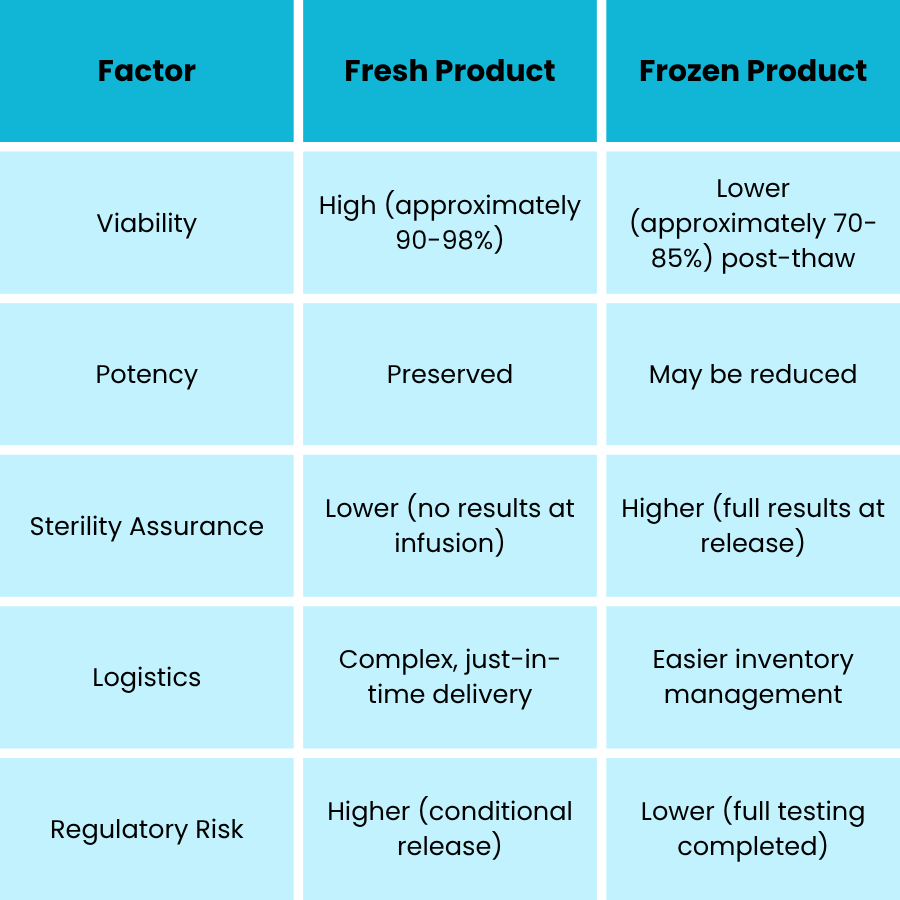
Cryopreserved Product – Full Release Panel Possible
Benefits
- Allows:
- Full 14-day sterility test
- Mycoplasma PCR
- Endotoxin
- Potency, identity, and viability
- Greater regulatory confidence in safety profile
Trade-Offs
- Viability loss due to freeze and thaw:
- Approximately 15 to 30 percent viability reduction in some cell types
- Potential potency loss (especially in T-cell therapies)
- May require:
- Cryoprotectants (such as DMSO), which have toxicity concerns
- Thaw logistics and specialized handling at clinical sites
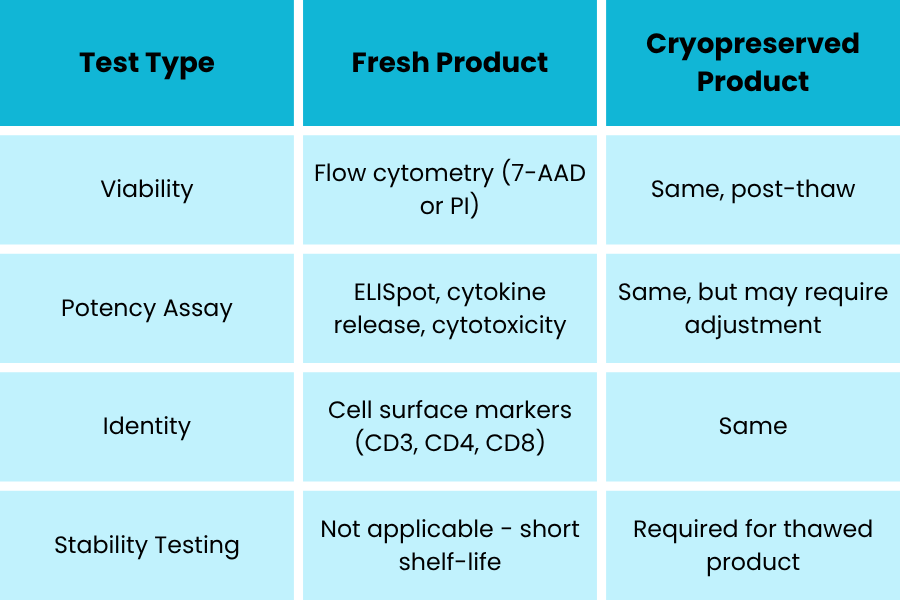
Global Solutions
Fresh autologous therapies offer maximum biological activity but pose regulatory and microbial risk due to limited release testing prior to administration.
Cryopreservation allows for full release testing but may compromise potency and viability, affecting clinical efficacy.
Outcome
Recommendation: Where sterility testing cannot be completed pre-release:
- Employ robust in-process controls
- Use rapid microbial detection methods
- Perform post-release sterility testing
- Justify release through a risk-based approach with regulatory authorities
Conclusions
GLOBAL supports clients to find the best approach for their products and submit to governing bodies successfully.
